AUSTIN, Texas — In the next two months, people in the U.S. could receive a Coronavirus diagnosis without having to leave the living room.
With long lines, crowded emergency rooms and a lot of worried people, getting a test currently isn't an easy process.
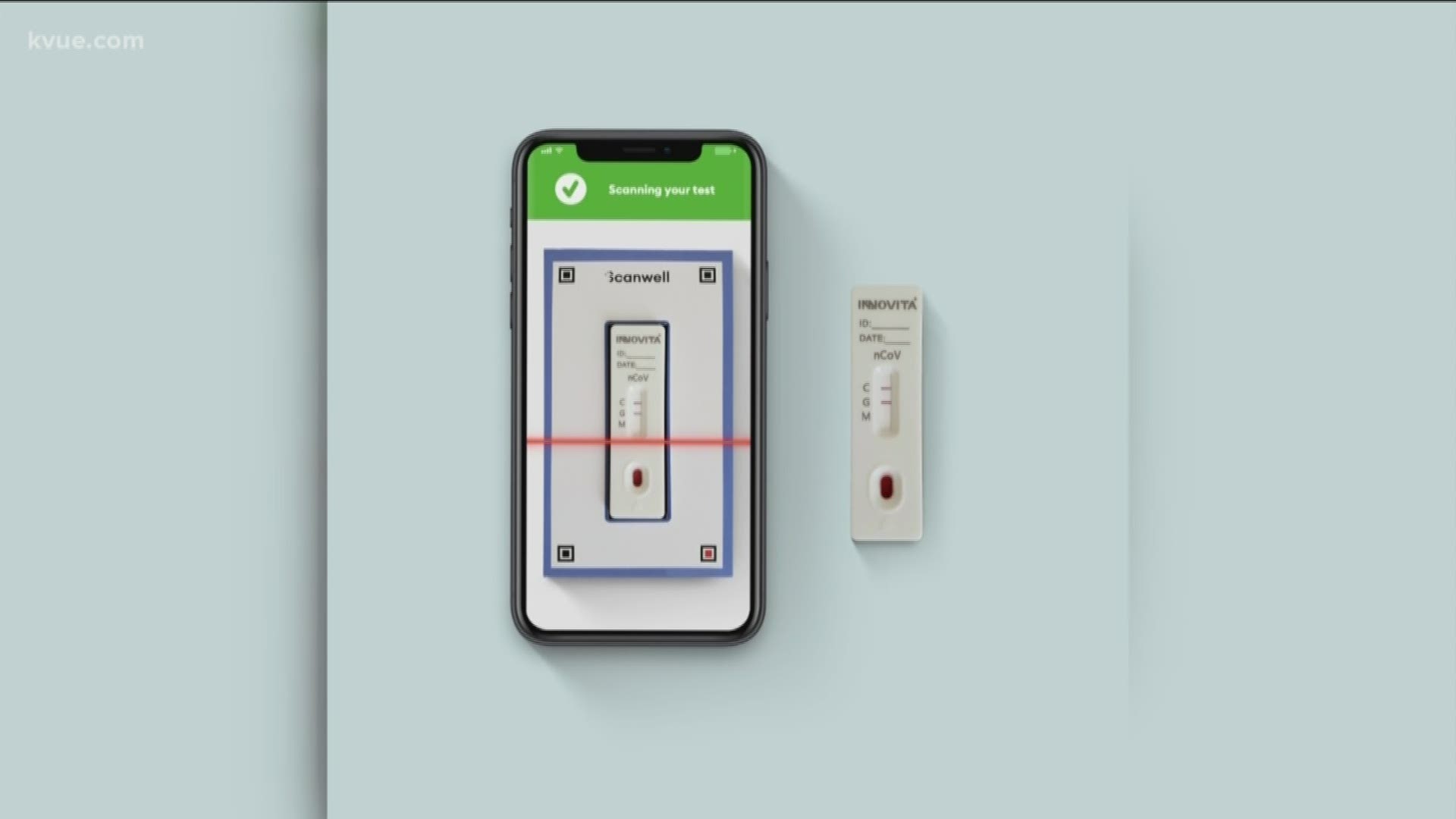
But Scanwell Health, a California-based company, is working to change that by launching the first at-home rapid COVID-19 test.
RELATED:
"It's a fingerprint blood test, one drop of blood and a 15-minute reaction time. Once that's complete, the results are sent back to the doctor," said Chief Medical Officer Jack Jang
Before the test is overnighted to a patient's home, Jang said they have to first describe the symptoms to a doctor online.
"A nurse or practitioner will determine if the patient meets the testing criteria by the CDC," said Jang.
He said Scanwell has been working closely with China
"We've been in contact with a manufacturing company in China called Anivita and, through those conversations, we realized that they already have a rapid blood test that we think we can adapt to be used at home," said Jang
RELATED:
If someone isn't currently experiencing COVID-19 symptoms, the test can detect if you've had the virus recently.
"A big advantage of the serology test unlike the PCR test, which is currently being used, this test can also tell you infections that have occurred months ago," said Jang.
Scanwell anticipates the rapid test kits will be available within six to eight weeks after getting approval from the Federal Drug Administration.
PEOPLE ARE ALSO READING: