AUSTIN, Texas — Children now make up more than 14% of confirmed COVID-19 cases in the U.S. since the pandemic began, according to the American Academy of Pediatrics.
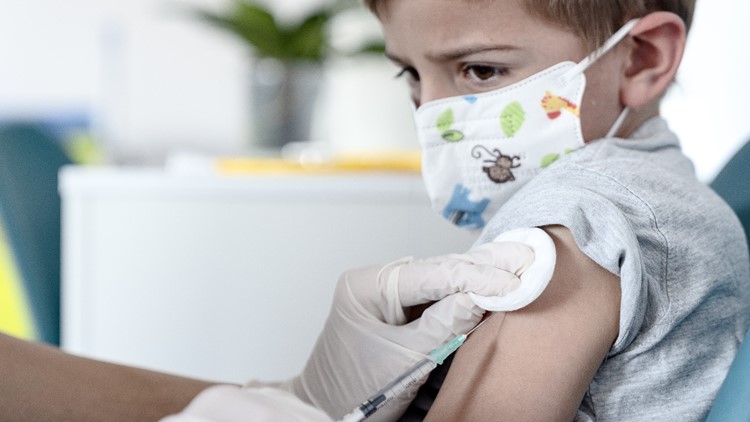
The Austin Regional Clinic is hosting Pfizer's new vaccine trial for children ages five through 11. While the study is two years long, we could likely see a new vaccine roll out even sooner if the trial sees success early on.
That's happy news for Austin-area parents, like Russell Schultz, who enrolled his 8-year-old son, Owen, in the trial.
"Yeah, he got his shot last Tuesday," said Schultz.
Schultz said his third-grader didn't have any symptoms besides a little case of the nerves and some soreness at the injection spot.
"He was definitely a little bit hyperventilation with the blood draw and the needles going in," said Schultz.
It's a blinded trial, which means none of the 25 participants know if they actually received the vaccine.
Dr. Gretchen Crook is one of the investigators for the children's trial. She's the principal investigator for the adult and adolescent trial.
She said there are two main differences between the trials. Dr. Crook said the children's trial is much smaller and more members are getting the vaccine.
"That more patients will be getting the vaccine percentage-wise, it's two to one who will get the vaccine versus placebo compared to one to one in the adolescent and adult trial," Dr. Crook said.
Dr. Crook explained the children's trial is smaller because they already learned so much from the adult and teen trials.
She said some of the side effects they have seen in the children's trial are the same as in the adult and teen trial – fever, body aches and chills.
"So the side effects of the vaccination that have been seen in the millions and millions of people who received the vaccine so far are consistent with typical side effects that are seen with vaccinations. And we're seeing the same thing in the children – fever, headache, chills, achiness, headache," she said.
Dr. Crook also said all the participants are followed very carefully.
"They are completing an electronic diary on a regular basis, at least weekly for their symptoms of infection," she said.
As for those critical of COVID-19 vaccines, Dr. Crook said researchers have been studying the technology behind them for years.
"So the way that the vaccine for the type that Pfizer is testing came out, it's really interesting because the mRNA vaccines actually have been studied for many years. And the intention before COVID was discovered was to develop an influenza vaccine using the mRNA technology, because it's a type of technology that is more easily interchangeable with the exact sequence of proteins that you're trying to protect against and so when it was determined that coronavirus, that COVID-19 was going to be a global pandemic, then the attention turns to finding a vaccine for this. And so it's building upon years and years of research that came before the type of vaccination was just fortunately very interchangeable," Dr. Crook said.
In the meantime, Schultz said living through the pandemic was hard enough.
"Well, certainly at the beginning you're wondering, are we all going to make it out of this?" Schultz asked.
Getting vaccinated and taking part in a vaccine trial is the least his family can do.
Enrollment for the study continues until the number hits 4,500 at the more than 100 sites worldwide.
Dr. Crook said she expects ARC will take a total of 40 to 50 members.
The trial will eventually expand to include children ages 2 years old to 5 years old, and 6 months old to 2 years old.
PEOPLE ARE ALSO READING: