AUSTIN, Texas — The KVUE Defenders are getting a first look at millions of problems with medical devices that remained secret for decades.
Since the beginning of the year, the Defenders have shared dozens of stories of patients' medical device problems – and now there is more proof that those people aren't alone.
Thousands of medical device complaints are kept in files at the FDA, accessible to the public. But there were 5.8 million other complaints, called "alternative summary reports," that remained hidden from the public for decades. Only the FDA had access to these files – doctors and patients could not see them.
RELATED:
These are the files that are now being released for the first time. They show two devices in particular caused millions of problems that doctors and patients never knew about.
Patients like Sandra Rush, who thought she had a rare cancer, a lymphoma that doctors linked to her breast implants.
“It changes your world, it changes your life,” she told KVUE.
The Defenders are now learning that cancer and other serious problems with breast implants are more common than patients like Rush were led to believe.
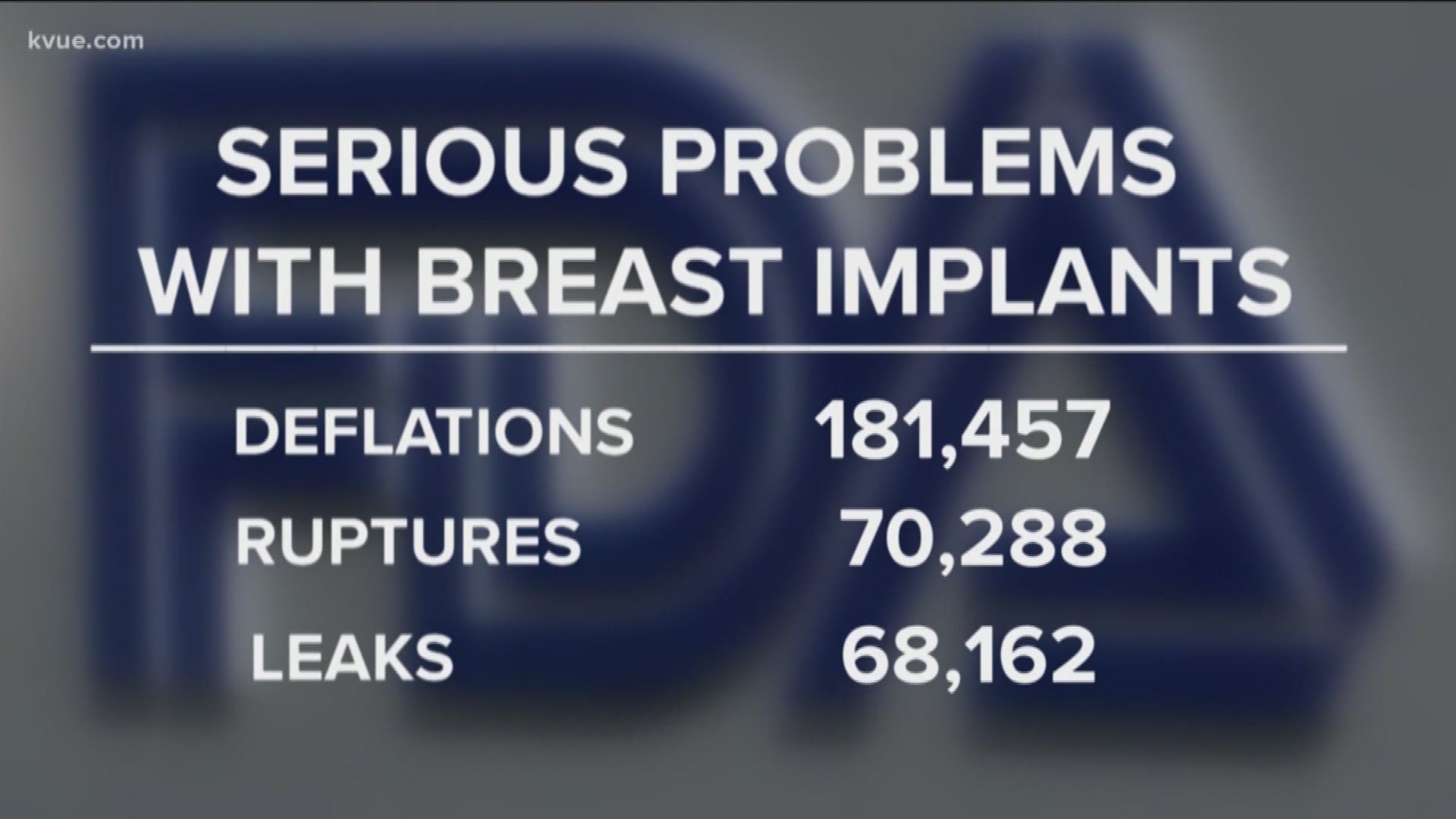
Much more than 70K breast implant issues
FDA reports originally showed 70,301 serious problems with breast implants. We now know there were 450,843 problems that we didn't know about – including 181,457 deflations, 70,288 ruptures and 68,162 leaks.
You can see the hidden reports the FDA released this summer at the FDA's website.
“We went a huge number of years without patients and physicians understanding the true risks of these products,” said Madris Tomes, a former FDA worker who started a company called Device Events to help people better understand medical device problems.
“Although the FDA released them, they didn't release them in a format that is usable by anybody except maybe an IT person,” Tomes said.
RELATED:
2.1M hidden dental implant issues
Dental implants had the most hidden complaints. There were 61,652 complaints we knew about and 2.1 million hidden reports.
“Most of them are coded as osseointegration issues, either failure to osseointegrate or loss of it. What that means is your body your jaw essentially didn't accept the implant,” Tomes said.
RELATED:
These hidden reports should help give patients a better understanding of the real scope of problems medical devices can cause. But the lesson here is you need to do your homework if someone tells you that you need surgery.
“Be informed,” Rush said.
“Talk to a few physicians, do research online, go on a Facebook group and find out what side effects people are having,” Tomes said.
KVUE has a Facebook group where people who have had issues with medical devices can share their experiences and resources with one another.
The FDA did not release outcome information, which means we don't know what happened to the devices after they malfunctioned or caused an injury – information the FDA does, in fact, release with pharmaceutical reports.
The FDA's response
The Defenders asked the FDA about that.
Brittney Manchester, the press officer for the FDA said:
"FDA did not include patient problem codes in the Alternative Summary Reporting (ASR) data released on its website this summer, and it has not been FDA’s practice to include patient problem codes in medical device reports (MDRs) released in the public MAUDE database because of concerns that they could be used to identify patients involved in the reported event.
KVUE: Overall what does the FDA have to say about the sheer number of reports in these summary reports? For instance, the number of medical device problems with dental implants appears to be much higher than previously known.
Manchester: "In the spirit of transparency, FDA made publicly available on its website data from adverse event reports submitted under the Alternative Summary Reporting (ASR) program from 1999 through 2019. These data reflect a small portion of all adverse events reported on medical devices during the time the program was in place. While these data were not posted on FDA’s website until June 2019, they have always been available to FDA analysts and have contributed to the agency’s broader ongoing efforts to monitor device performance, detect potential device-related safety concerns or signals and contribute to the benefit-risk assessment of these products. We can and have used these data to inform our current and past surveillance efforts.
Dental implants are commonly-used medical devices, and a high volume of reported events is expected. The number of dental implant companies and marketing submissions for dental implants have increased over the past 20 years. Likewise, the use of, and number of patients receiving dental implants has also increased each year during this same time period and continues to go up. We attribute the counts observed in the data largely to increases in both product availability and use as well as FDA’s efforts to improve the reporting of adverse events and malfunctions for all medical devices."
KVUE: The FDA did away with the adverse summary reports and began a new program this summer called Voluntary Malfunction Summary Reporting Program, which critics say is virtually the same as ASRs. Can you tell me overall (systemwide) how many VMSRs have been reported to the FDA since implementation?
Manchester: "FDA has received approximately 2,800 reports to date under the Voluntary Malfunction Summary Reporting (VMSR) program. As outlined in 83 FR 40973, manufacturers submit these reports on the same Form 3500A that is used for reporting of individual events. For VMSR program submissions, manufacturers must include the number of reportable malfunctions the report represents in the narrative section of the form. All information FDA receives through submission of this form – whether it reflects an individual reportable event or a VMSR submission – is made available to the public in MAUDE after being redacted for consistency with applicable disclosure laws, regulations, and policies."
PEOPLE ARE ALSO READING: