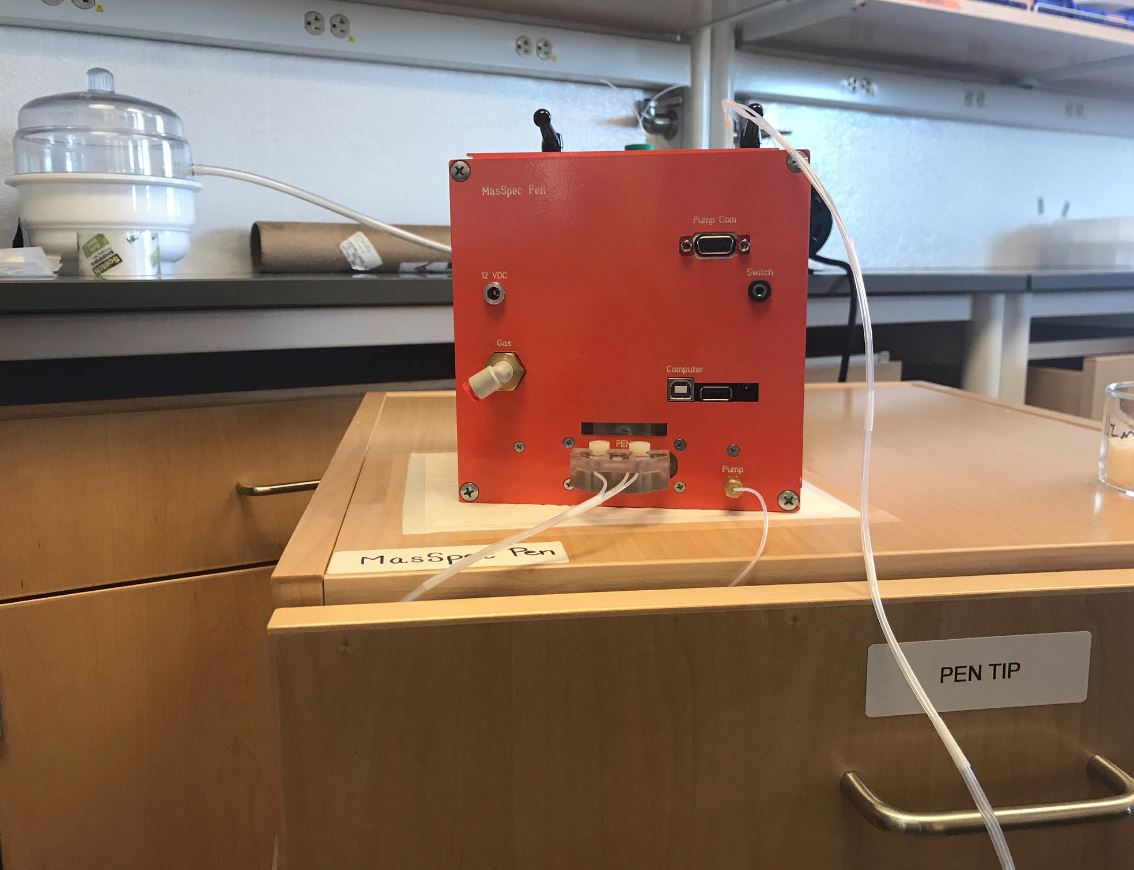
At first glance, the orange contraption in the middle of the high-tech lab could easily be confused for a Jack-in-the-box, seemingly out of place surrounded by scientific equipment.
But it's no toy.
"We've developed a handheld probe that can diagnose cancer by touching the tissue," explained Liva Eberlin, an assistant professor in the Department of Chemistry at UT who's helped lead the creation of the MasSpec pen.
Here's how it works:
The pen drops a small drop of water onto the tissue, which extracts biomolecules.
Those biomolecules are then run through a mass spectrometer, which diagnoses the probability of cancer.
The whole process takes just 10 seconds.
"The current technique actually in practice is called pathology or histopathology. That takes about 30 minutes to get a response," explained Eberlin.
During surgery - that time difference is crucial.
"For the patients, this is really good because the longer they're exposed, there's higher risks for being under anesthesia," Eberlin explained.
Another component: increased accuracy.
The team wrote software to train the machine to identify the probability of cancer in respective tissue.
"These are the pen tip molds, and we have varying sizes from .6 millimeters to 5 millimeters, so depending on the surgery you can use the appropriate pen tip size," explained Kyana Garza, a graduate student working on the project.
"Everything is disposable. So it's plug in and plug out," added Jialina Zhang, a research associate. "For the pen tip, we have a design which can (prevent) crossover-contamination from the former analysis."
In tests on mice and extracted human tissue, early results show a 96.4 percent accuracy rate with MasSpec, compared to 80-90 percent with current practices.
"We're talking about thousands of lives," said Eberlin.
Researchers believe it can have far-reaching benefits throughout the healthcare industry.
"There's this aspect of the emotional impact for a patient to know that their cancer came back, that we can probably be helping with. But also for the healthcare system, the financial burden of second and third surgeries can really be reduced," said Eberlin.
The research team has worked for the past two years to get to this point.
"We're still in the testing phase. We've demonstrated feasibility, we've looked at 253 human tissue samples, and we've also during mice surgery, so in-vivo (in living animals), and it's working really well. Our next steps are to validate the technology with a larger sample size, and then early next year we're looking to start our clinical studies, pilot studies, in collaboration with our clinicians and surgeons in the Texas Medical Center," said Eberlin.
The process to test on humans during surgery will take about a year. From there, it will need to go through the FDA regulation process.
While they have not set a price for market on the technology, they have begun to learn about it.
"We've done a lot of market assessment to see if there will be an interest. The technique would be cheaper than some large instruments at hospital, like MRI (machines) or CAT scans, but it would be an investment. So we don't have values right now, but we really think it would be something that even though it's not cheap, it would be accessible, and a very good investment," said Eberlin.
To learn more, click here.