AUSTIN, Texas — The Food and Drug Administration is recommending all breast implants come with the highest level warning the agency issues, a big change in how they are being sold across the nation.
The so-called "boxed warnings" would require labels that read "breast implants are not considered lifetime devices" and "breast implants have been associated with the development of a cancer of the immune system called breast implant-associated anaplastic large cell lymphoma."
The recommendation also includes making sure patients receive a booklet/brochure, patient decision checklist, this boxed warning and patient device card.
According to the FDA, “Specifically, FDA believes manufacturers should include a boxed warning and patient decision 200 checklist to help ensure patients receive and understand information about the benefits and risks 201 of breast implants.”
RELATED:
The patient checklist would include these items:
- Situations in which the device should not be used or implanted
- Considerations for a successful breast implant candidate
- Risks of undergoing breast implant surgery
- Importance of appropriate physician education, training and experience
- Risk of BIA-ALCL and systemic symptoms
- Discussion of options other than breast implants

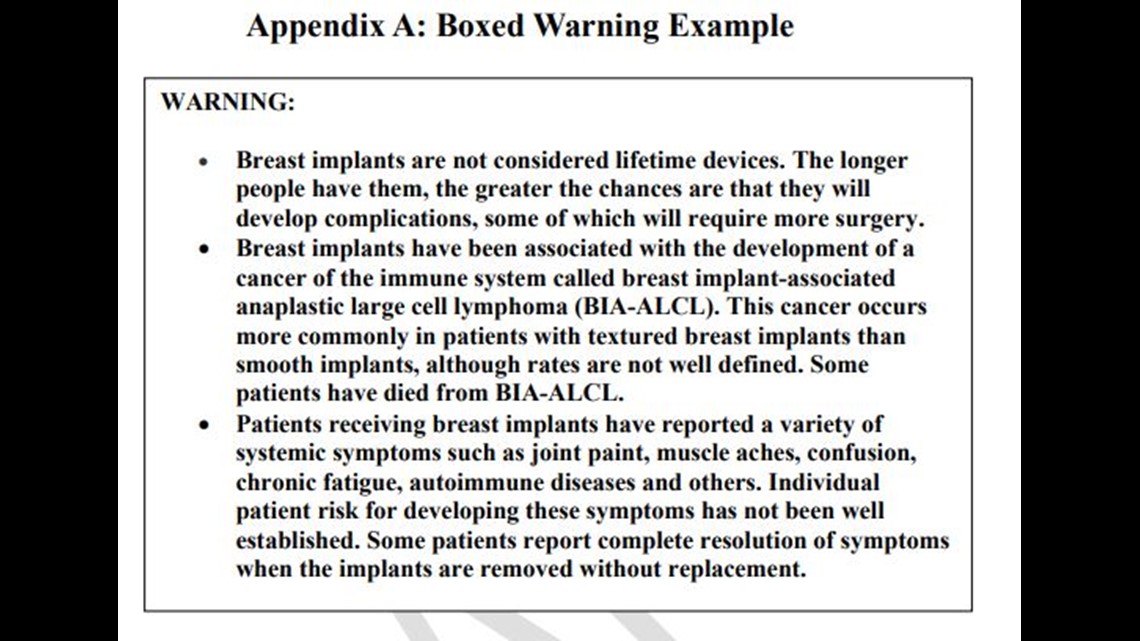
The wording still needs final approval, but the hope is this will be a step toward making sure patients get information about the real risks of getting breast implants.
This is one of the stories the KVUE Defenders have been reporting on since the beginning of the year with our Medical Device Dangers series.
You can watch the full series here.
PEOPLE ARE ALSO READING: